 "kanadanmajava1" (kanadanmajava1)
"kanadanmajava1" (kanadanmajava1)
08/10/2016 at 14:57 • Filed to: None
 1
1
 12
12
 "kanadanmajava1" (kanadanmajava1)
"kanadanmajava1" (kanadanmajava1)
08/10/2016 at 14:57 • Filed to: None |  1 1
|  12 12 |
My Packard has a weird 6V (lead acid) battery. It’s a type that cannot be found from here so I’m slightly reluctant to change it.
It has a problem. It’s constantly boiling. At first I thought that it was related to Packard’s the voltage regulator. The target voltage was a bit too high. I tuned it down but the battery kept on boiling. After I fetched the battery from the car and charged it with a smart charger I noticed that the boiling is indeed constant. When it boils it somehow makes the fluid escape from the venting caps and it leaks out from the battery. The fluid finds its way over the lid whether the caps are on or off.
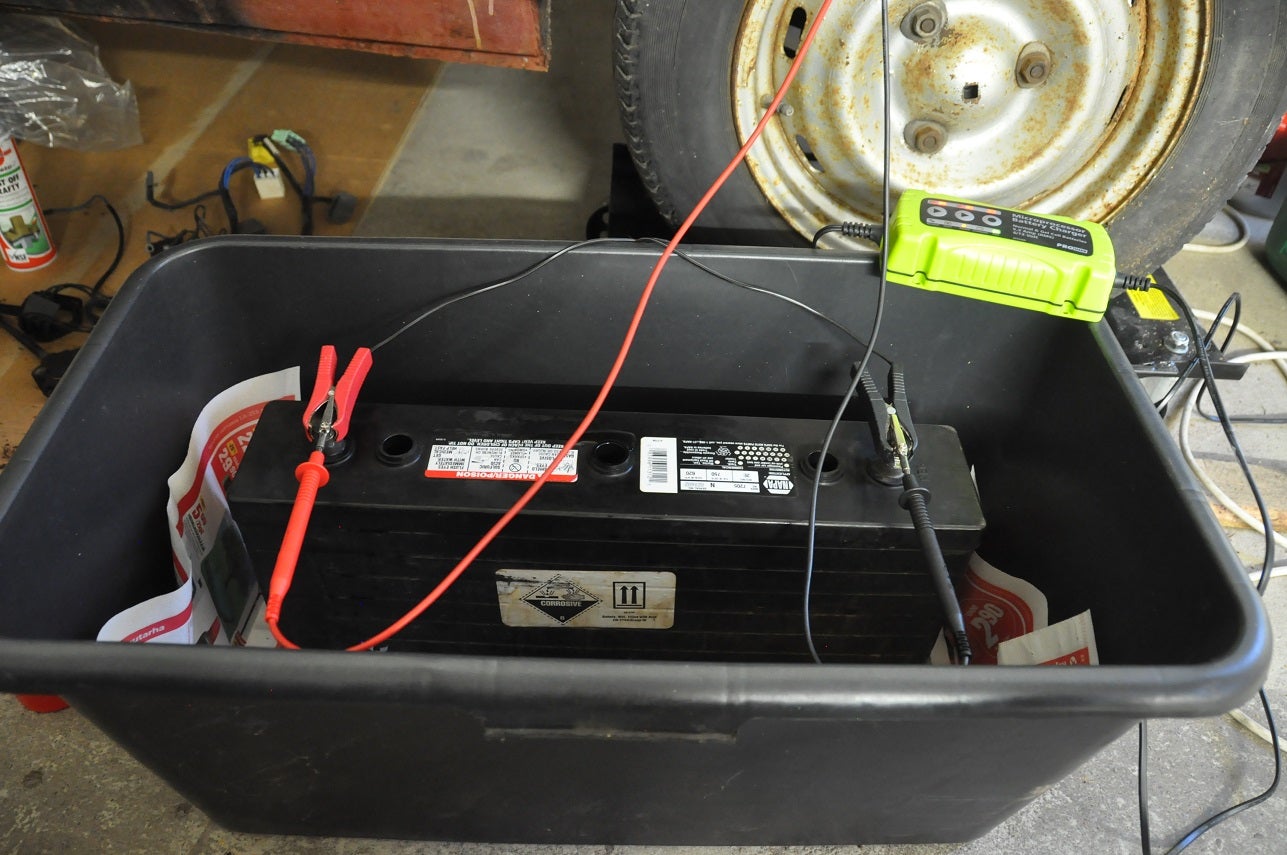
Three months ago I took the battery off from the charger. The battery just kept on boiling. This has now continued without a pause and the battery has been messing up my garage floor.
Yesterday I cleaned the the battery from outside and placed it in a plastic box with some newspaper to see how the boiling happens. Before I did anything the voltage was 6.1 V. I noticed that the middle cell was quite empty but the others were still full. I also shook and tilted the battery as I found some rumors that the electrolyte can have uneven mix if the battery has been empty at some point. It was quite empty at some point.
Now it has been in charge for more than one day and the charger thinks its full. The middle cell started pushing out fluid after couple of hours of charge and it has been doing it since. The voltage was 6.7 V immediately after I disconnected the charger but it seems to dropping.
Does someone have any ideas what is happening with the battery?
I have added only distilled water but the former owner might have slipped in something other by mistake. I couldn’t find any info what could happen if a lead acid battery meets some other fluid.
 just-a-scratch
> kanadanmajava1
just-a-scratch
> kanadanmajava1
08/10/2016 at 15:12 |
|
Is the battery hot? If not, it’s probably not boiling. I suspect you may have some hydrolysis going on.
Hydrolysis means your battery is discharging into the water and separating the oxygen and hydrogen. A small internal short of some kind would do this. I'm not an expert in batteries. There may or may not be a fix for this.
 Scott
> kanadanmajava1
Scott
> kanadanmajava1
08/10/2016 at 15:13 |
|
Yes sounds like your electrolyte is indeed low, so basically your have a lead and water battery not a lead acid battery. I might be thinking of something else but it seemed like you used to be able to get a tester, similar to testing your coolant, to see if your electrolytes are low. I doubt you can buy any electrolytes. I suspect the answer would be you have to replace your battery. If it is possible to add more electrolytes then I should think an autoparts store could do it. I’m sure it’s possible, but acid is generally something too dangerous to sell to the general public.
You might look for an 8V battery, I seem to recall the guys on wheeler dealer doing that because they could not get a 6V battery and 8 was close enough. Although when I saw that I though it odd as I have seen 6V batteries but had never seen or heard of an 8V, so you may be out of luck either way.
One other thought, probably not worth the effort, but look into several motorcycle batteries, I think 6V are somewhat more available in motorcycles than cars, you'll just need to pair enough together.
 Scott
> just-a-scratch
Scott
> just-a-scratch
08/10/2016 at 15:20 |
|
That reminds me... be careful. Batteries put out hydrogen when charging... that’s why you always want a battery in a vented storage. If hydrolysis is going on, and the short may just be too much water, they your producing even more hydrogen and oxygen, and if you get a spark with the right mixture of oxygen and hydrogen you'll get a big bang too.
 RamblinRover Luxury-Yacht
> Scott
RamblinRover Luxury-Yacht
> Scott
08/10/2016 at 15:21 |
|
“acid is generally something too dangerous to sell to the general public”
 RamblinRover Luxury-Yacht
> just-a-scratch
RamblinRover Luxury-Yacht
> just-a-scratch
08/10/2016 at 15:22 |
|
This is most likely it. An internal battery fault.
 Die-Trying
> Scott
Die-Trying
> Scott
08/10/2016 at 15:22 |
|
i have heard the “old ones” speak of getting a 12v battery, and putting a screw, or tapping a bolt into the center( from the top), and making it a 6volt battery that way.........
 Scott
> RamblinRover Luxury-Yacht
Scott
> RamblinRover Luxury-Yacht
08/10/2016 at 15:25 |
|
OK... I did say I doubt... not you can't... cause I have not seen it until now. now I know.
 RamblinRover Luxury-Yacht
> Scott
RamblinRover Luxury-Yacht
> Scott
08/10/2016 at 15:28 |
|
No worries. Nitric acid, hell yes there are some limits on because EXPLODEY THINGS, but sulfuric... not so much. Nor hydrochloric (“muriatic”) - you can buy that shit at Wal Mart.
 kanadanmajava1
> Scott
kanadanmajava1
> Scott
08/10/2016 at 15:28 |
|
I think acid-water mix is sold here (in 37% concentration). I could try to drain it and add new fluid.
Getting anything that would fit in the original place doesn’t seem to be possible from any shop known to me. The battery type is a “group 2E” battery and some farming equipment seems to be still using it in the US. I think some other cars besides Packard used it too but not since mid 50's. The specs are:
Cold Cranking Amps (CCA): 620 CCA
Cranking Amps (CA): 765 CA
Reserve Capacity (AH): 190Ah
Length (In): 19-1/4 Inch
Width (In): 4 Inch
Height (In): 8-7/8 Inch
Weight (Lbs): 42 Lbs.
So the dimensions are pretty stupid. If I would have to change to some other type I would likely have to place in the trunk or hide it inside the front fenders.
 kanadanmajava1
> just-a-scratch
kanadanmajava1
> just-a-scratch
08/10/2016 at 15:31 |
|
It doesn’t seem be generating any heat. It was able to constantly boil for couple of months before losing the voltage completely so the boiling doesn’t seem to sourced from the charge.
 kanadanmajava1
> Scott
kanadanmajava1
> Scott
08/10/2016 at 15:36 |
|
Yeah. I have been pretty careful. One of my colleagues exploded a battery over his face years ago and he still has a small blind spot in one of his eyes. Hydrogen explodes with very weird mixtures so any amount of it is eager to explode.
I never remove the charging connectors while the charger is charging. Even though batteries shouldn’t boil with smart chargers I have found one annoying exception.
 JawzX2, Boost Addict. 1.6t, 2.7tt, 4.2t
> kanadanmajava1
JawzX2, Boost Addict. 1.6t, 2.7tt, 4.2t
> kanadanmajava1
08/11/2016 at 17:29 |
|
Wire multiples (3) in parallel to get the amp rating you need...?
https://www.amazon.com/Shorai-LFX18L2…
should be miles of space left over. you could even hide them *INSIDE* a hollowed out shell of the correct battery...